Aurore Palmaro, MSca, Raphael Bissuel, MDb, Nicholas Renaud, MSca, Geneviève Durrieu, PharmD, PhDa, Brigitte Escourrou, MDb, Stephane Oustric, MDb, Jean-Louis Montastruc, MD, PhDa, Maryse Lapeyre-Mestre, MD, PhDa
Abstract
OBJECTIVE: To study the characteristics of off-label prescribing and adverse drug reaction (ADR) occurrence in a sample of pediatric outpatients treated by general practitioners.
METHODS: A survey on pediatric drug prescribing was implemented in 46 general practices in southwestern France. All consecutive patients aged 0 to 16 years were included. Patient characteristics, reasons for consultation, and drug prescribed (including indications) were collected. ADRs occurring #10 days after the date of consultation were recorded by the general practitioners (spontaneous notification). Off-label prescription was defined as prescribing outside the specifications of the Summary of Product Characteristics.
RESULTS: Among the 2313 children seen between March 8, 2011 and July 31, 2011, 1960 were exposed to >1 prescribed drug. Mean age was 5.6 years, with a gender ratio of 1.1. Among children with prescriptions, 37.6% (n = 736) were exposed to >1 off-label prescription and 6.7% (n = 132) to >1 unlicensed drug. Off-label prescribing involved an unapproved indication in 56.4% of cases (n = 416), a lower dosage (26.5%, n = 195) or higher dosage (19.5%, n = 144) than specified, age not labeled (7.2%, n = 53), incorrect route of administration (3.5%, n = 26), and contraindication (0.3%, n = 2). A total of 23 ADRs were reported (1.5% of patients with off-label prescriptions). ADR occurrence was not significantly related to off-label drug prescribing.
CONCLUSIONS: Despite the numerous initiatives implemented for promoting rational medicine use in children, the prevalence of off-label prescription in outpatient pediatric practice remains high.
a Laboratoire de Pharmacologie Médicale et Clinique, Centre Midi-Pyrénées de PharmacoVigilance, de Pharmacoépidémiologie et d’Informations sur le Médicament, Equipe de Pharmacoépidémiologie, INSERM U1027, Centre Hospitalier Universitaire, Faculté de Médecine, Université de Toulouse, France; and bDépartement Universitaire de Médecine Générale des Facultés de Médecine de Toulouse, France
Miss Palmaro performed statistical analyses, interpreted the findings, and drafted the initial manuscript; Dr Bissuel, Mr Renaud, Dr Escourrou, and Dr Oustric designed the data collection forms, coordinated and supervised data collection, and revised the article; Drs Durrieu and Montastruc interpreted the data and critically reviewed the manuscript; Dr Lapeyre-Mestre conceptualized and designed the study, interpreted the findings, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
WHAT’S KNOWN ON THIS SUBJECT:
Off-label prescribing in children has been widely described. There has been growing awareness and action from regulatory bodies since 2006 to promote drug assessment in children and rational prescribing.
WHAT THIS STUDY ADDS:
In comparison with a similar study done in 2000, there was no significant change in off-label prescribing in children. In contrast with the previous findings, off-label prescribing did not increase risk for adverse drug reactions.
Off-label prescribing has been widely observed in children. However, administration of a drug outside the conditions assessed during clinical trials may result in adverse drug reactions (ADRs).1–3 Moreover, the specificity of children’s physiology and pharmacology should not be neglected.4 For years, the lack of drug evaluation in children has been evoked as a factor favoring off-label prescribing. More recently,5 there have been several incentives to promote drug assessment in children, including promotion of good prescribing practice for antibiotics. In Europe, Pediatric Regulation No. 1901/2006 came into force in 2007. As stated by Lehmann,6 “The Regulation (EC) No 1901/2006 sets up a system of requirements, rewards and incentives, together with horizontal measures, to ensure that medicinal products are researched, developed and authorised to meet the therapeutic needs of children.” This regulation focuses on both public actors (European member states), encouraging them to promote research and pediatric drug availability, and pharmaceutical companies (incentives to conduct clinical trials in children). These actions tend to demonstrate that issues related to drug assessment and prescribing in children have raised awareness among policymakers. Despite regulatory differences, off-label prescribing is an issue in both the United States7 and Europe. As a result of these initiatives, it may be hypothesized that prescribers would be more aware of the potential consequences of off-label prescribing, decreasing the prevalence of off-label prescribing and associated risks.8 A study2 performed in the early 2000s in France showed that off-label prescribing was highly prevalent (42%) and significantly associated with ADR occurrence. Since this period, in line with the promotion of drug evaluation and the development of new pediatric formulations, the situation is likely to have evolved. To obtain an overview of the current situation 10 years after this study,2 and considering that off-label prescribing in children has been poorly investigated in the ambulatory care setting, we designed a similar survey on the consequences of off-label prescribing in children. Our aim was to study the characteristics of off-label prescribing and ADR occurrence in a sample of pediatric outpatients treated in ambulatory practice. The study by Horen et al2 was performed among pediatricians, whereas the current study was implemented among general practitioners (GPs). This difference reflects an evolution in pediatric practice in France, where ∼3 out of 4 children are currently referred to GPs.9 This shift occurred in response to the decrease in the number of pediatricians in France. Difference in prescribing practices between French GPs and pediatricians have already been described.10 Children treated by pediatricians receive fewer antibiotics than those managed by GPs but also fewer corticosteroids and significantly fewer antiinflammatory drugs. Expectorants and digestive motility drugs are also less likely to be prescribed by pediatricians than by GPs.10
METHODS
Study Design and Settings
A survey on pediatric drug prescribing was implemented in a sample of GPs from practices in the Midi-Pyrénées area (southwestern France). GPs were recruited from among those involved in the resident training program of the general practice university department. To be involved in resident training, they must have followed a complementary course organized by the department.
Participating GPs prospectively recorded information from all consecutive consultations involving patients aged 0 to 16 years for 1 week. Patient characteristics (age, gender, weight), main reasons for consulting, and drug prescribed (including dosage, route, and indication) were collected. GPs were provided forms that they completed electronically after each visit.
Children were categorized into 3 groups: newborn infants (0–27 days) and toddlers (28 days–23 months), grouped together because of sample size; children (2–11 years); and adolescents (12–16), according to the International Conference on Harmonization guidance E11.11
Prescribing Patterns
To assess representativeness of prescribing patterns of participating GPs, we obtained data from the national health insurance database (Système National d’Information Inter Régimes de l’Assurance-Maladie)12,13 concerning all drugs prescribed and reimbursed for infants aged 0 to 16 years in the same area and during the study period. The health insurance database contains basic patient demographics, prescriber’s specialty, drug prescribed, and quantity dispensed, but the indication is not available.
Off-Label Prescribing
Off-label prescription was defined as prescribing outside the specifications of the Summary of Product Characteristics (SPC)14, equivalent to the prescribing information in the United States. As was done previously,2 off-label prescribing was defined according to 9 categories, including prescribing a drug withdrawn from the market, contraindications, different indications, age, different route of administration, higher dose or lower dose than recommended, and inadvisable coprescribing. To determine off-label status, we referred to the SPC. If the indication was not cited in the
paragraph “Therapeutic Indications,” the drug was considered off label for “different indication” (ie, unlabeled indication). However, for antibiotics, where the indication specified by the physician was a viral infection (rhinopharyngitis, laryngitis, tracheitis), the indication was considered labeled, with the assumption that the prescriber suspected a bacterial superinfection. To determine off-label dosage, we applied a 20% tolerance margin to the prescribed daily dose. For symptomatic treatments such as cough syrups, a lower number of doses compared with recommendations was not considered off label. For contraindications and inadvisable coprescriptions, we referred to the corresponding section of the SPC. Unlicensed drugs were those with no valid marketing authorization at the time of the study. Homeopathic preparations were considered products without license. Only 1 off-label category was assigned to each drug prescribed.
In case of multiple possible categories, the status leading potentially to more damage was selected (namely, by decreasing order of “priority”: contraindication or different indication, age, route of administration, higher dose or lower dose than recommended, and inadvisable coprescribing). For all analyses, children were considered exposed to off-label prescribing if ≥1 of the drugs in the prescription form was off label. So even if children could potentially be exposed to ≥1 drug or ≥1 off-label drug, all percentages were calculated with the number of children as the denominator, including drug exposure and off-label drugs.
ADR Occurrence
Each GP was asked to identify and report to the regional pharmacovigilance center any ADR occurring ≤10 days after the date of consultation. ADRs were classified according to the Medical Dictionary for Regulatory Activities lower-level term and primary system organ class. All suspected ADRs were classified into 4 causality levels (ie, “possible,” “plausible,” “likely,” and “very likely”), according to the French ADR causality assessment method.15 A “serious” ADR was defined as “any untoward medical occurrence that at any dose results in death, requires hospital admission or prolongation of existing hospital stay, results in persistent or significant disability/incapacity, or is life threatening.”16 An “unlabeled” or “unexpected” ADR is a reaction whose nature or severity is not consistent with data contained in domestic labeling or market authorization or expected from the characteristic of the drug.16
Confidentiality and Ethics
During recruitment, GPs already involved in training residents were proposed to participate in this study by the general practice university department. Only 1 declined to participate. A written consent form was not required for GPs. All data were treated anonymously regarding the patients and GPs. Prescriber and patient IDs were assigned to match physician–child pairs. All ADRs were reported to the regional pharmacovigilance center, according to French pharmacovigilance good practices.17 Access to reimbursed drug data on the health care database was allowed through authorization for evaluation of health care practices no. 1516745 of the French Data Protection Authority (Commission Nationale Informatique et Libertés).
Institutional review board approval was not required for this observational study.
Statistical Methods
Sample Size Calculation
According to the study performed in 2000 in the same area,2 where 42.3% of patients were exposed to off-label prescribing, with a relative risk and ADR occurrence of 3.44, the sample size for the current study would have to be 922 patients to detect a risk increase of 3.44 in ADR occurrence with an 80% power (α = .05).
Data Analysis
Descriptive analyses included mean values ± SDs for quantitative variables and frequencies and percentages for qualitative variables.
We performed a bivariate analysis by using Pearson’s x2 test or Fisher’s exact test for qualitative variables and Student’s t test or Mann–Whitney parametric test for quantitative variables. Variables (age class, gender, number of medications prescribed, and reason for consultation according to International Classification of Diseases, 10th Revision blocks) were entered into a multivariate logistic regression model, with offlabel prescribing as the dependent variable. Crude and adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated. We investigated the interaction between variables by using the log likelihood ratio test. A significance level of .05 was used. We performed statistical analyses by using SAS 9.3 software (SAS Institute,
Inc, Cary, NC).
Results
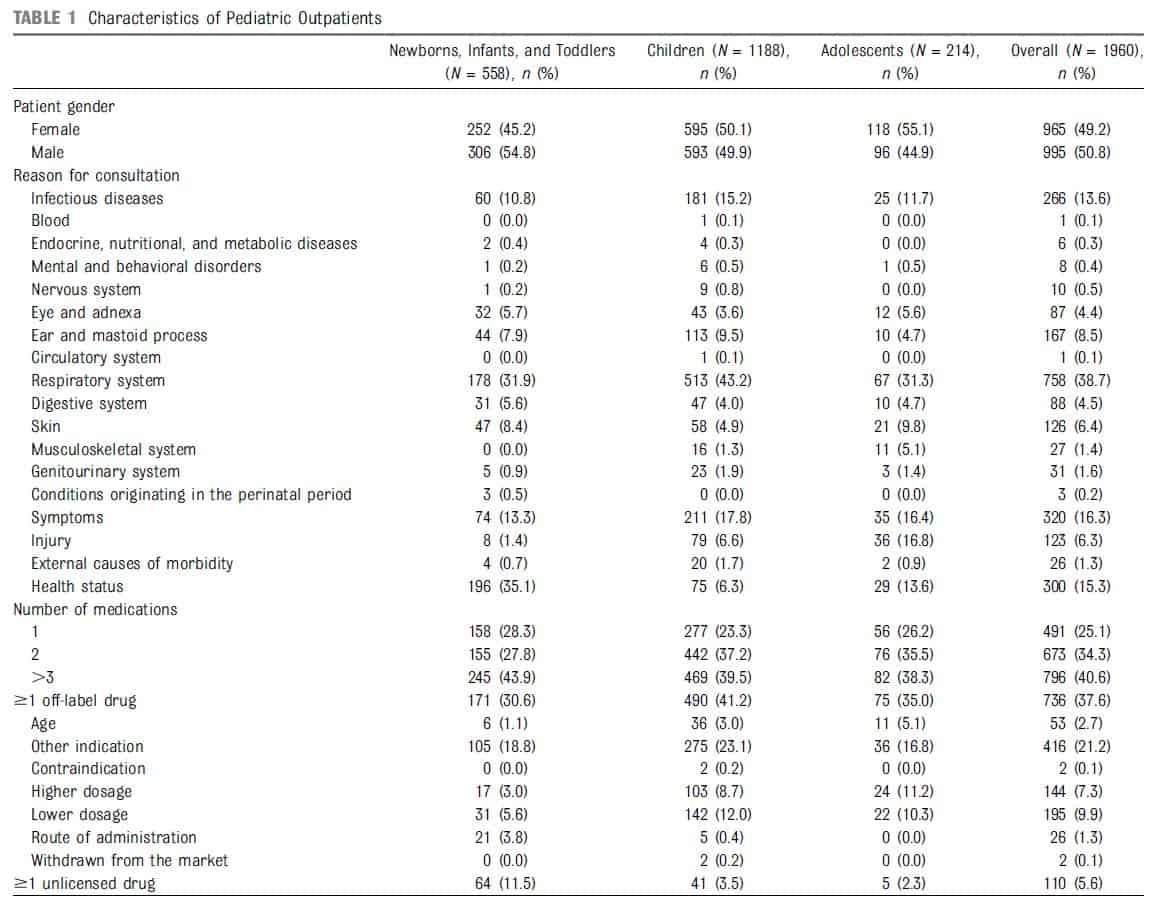
Children Characteristics
From March 8, 2011 to July 16, 2011, 2313 patients were recruited by 38 GPs. Among them, 1960 received ≥1 prescribed drug. All GPs prescribed ≥1 drug. Mean age was 5.6 years (SD 4.5, range 0–16), with a gender ratio of 1.1. Among the reasons for consultations (diagnosis, symptoms, or referral), diseases of the respiratory system (acute tonsillitis, n = 224; acute nasopharyngitis, n = 220), factors influencing health status (health checkup, n = 134; and immunization, n = 131), and symptoms (eg, fever) were the most commonly represented (Table 1). Mean number of drugs prescribed was 2. Analgesics (52% ever exposed, n = 1003), antibacterials (22%, n = 422), and antiinflammatory and antirheumatic products (18%, n = 345) were most represented (Table 2). Paracetamol, ibuprofen, and amoxicillin were most common.
Pattern of Prescribing From Health Insurance Data
In 2011, there were 2971 GPs and 125 pediatricians in the Midi-Pyrénées area. During the study period, 319 857 infants aged ≤16 years were reimbursed for ≥1 drug prescribed. GPs represented 77% of overall drug prescriptions (pediatricians 17%). To indicate the representativeness of prescribing patterns, drug classes from GPs in our study, together with those from GPs in the health insurance database, are presented Table 2. Excluding analgesics (essentially paracetamol) that were less frequently prescribed than in our sample (37.7% vs 51.9%), prescribing patterns were quite similar, with the same prescribed Anatomical Therapeutic Chemical (ATC) classes in the top 5.
Off-Label Prescribing
Among the 1960 children receiving ≥1 drug, 37.6% (n = 736) were exposed to ≥1 off-label drug (638 with 1 drug, 94 with 2, and 4 with 3 off-label drugs) and 7.3% (n = 143) to ≥1 unlicensed drug. Off-label prescribing involved an unapproved indication in 56.4% (n = 416), a lower dosage in 26.5% (n = 195), a higher dosage in 19.5% (n = 144), age not labeled in 7.2% (n = 53), incorrect route of administration in 3.5% (n = 26), and contraindication in 0.3% (n = 2). Nasal decongestants (tixocortol, a corticosteroid, and tuaminoheptane, a sympathomimetic), H1 antihistamines mequitazine, a phenothiazine derivative, and desloratadine), and corticosteroids (betamethasone and prednisolone) were the most frequently involved in off-label prescribing for indication. According to the SPC, tixocortol is indicated for nasopharyngeal inflammatory and allergic conditions (allergic rhinitis, seasonal rhinitis, acute and chronic congestive rhinitis, vasomotor rhinitis), whereas tuaminoheptane is approved for nasopharyngeal diseases with excessive mucus secretion in patients >15 years old. Tixocortol was prescribed with an unapproved indication for common rhinitis, whereas it is indicated only for allergic, congestive, or vasomotor rhinitis.
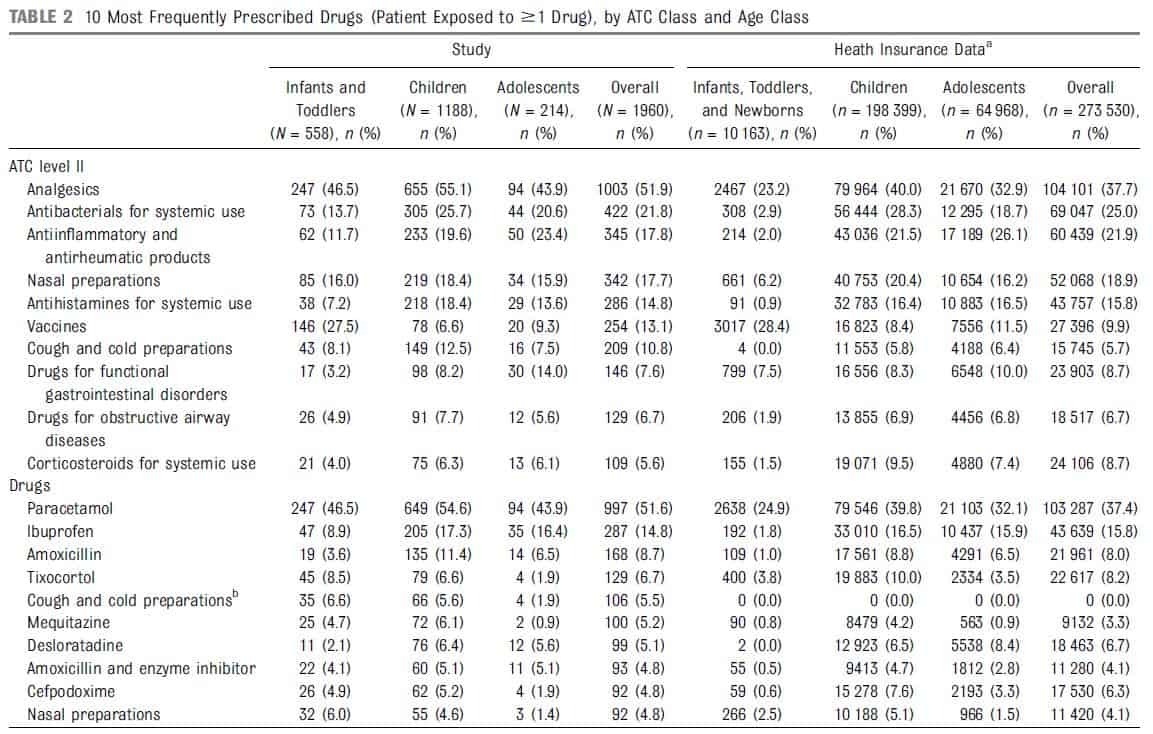
Data derived from regional health insurance data (France, GPs only, prescriptions from March 8, 2011 to July 16, 2011).
a Prevalence of patients exposed to ≥1 drug from health insurance data could be derived from >1 prescription per patient.
b Not reimbursed by the French health insurance.
Mequitazine and desloratadine were prescribed for cough, although they are indicated for the relief of symptoms associated with allergic rhinitis, urticaria, or conjunctivitis (mequitazine only). Prednisolone and betamethasone were prescribed with unlabeled indications for cough, bronchitis, or laryngitis. Drugs with lower dosage than specified involved mainly cold preparations (n = 24); ibuprofen (n = 20); helicidine, a cough suppressant (n = 19); and rifamycin for topical use (n = 17), whereas drugs with higher dosage than specified (n = 144) were represented mainly by tuaminoheptane (n = 39), phloroglucinol (n = 20), and amoxicillin (n = 17). When age was unlabeled (n = 53), antiinflammatory drugs (ibuprofen, diclofenac, and tiaprofenic acid, n = 12), antiinflammatories for topical use (diclofenac, niflumic acid, n = 7), analgesics (paracetamol and tramadol, n = 7), and antidiarrheals (medicinal charcoal, Saccharomyces boulardii, racecadotril, n = 6) were most frequently identified. An incorrect route of administration (n = 26) was encountered exclusively for vaccines and primarily (n = 23) for measles vaccine (in combination with mumps and rubella), which was administered by intramuscular injection, although the SPC recommend subcutaneous administration. Physiologic serum (n = 63) and oral rehydration solution (n = 13) (mainly for infants and toddlers) accounted for a large proportion of unlicensed drugs (n = 143).
Details of active substances and type of off-label prescribing are provided in Table 3.
Determinants of Off-Label Prescribing
Determinants of off-label prescribing, identified through multivariate logistic regression, are presented in Table 4. Number of medications >3 (adjusted OR 5.65; 95% CI, 4.29–7.43; P < .0001) and ophthalmologic consultations (adjusted OR 1.62; 95% CI, 1.02–2.57; P < .0406) were more likely to be associated with off-label prescribing. Conversely, some conditions are more likely to exhibit a protective effect, such as checkups and immunizations (adjusted OR 0.21; 95% CI, 0.15–0.29; P < .0001) and symptoms (adjusted OR 0.69; 95% CI, 0.53–0.92; P <.0096).
ADR Occurrence
Among children receiving ≥1 drug, 23 presented with ≥1 ADRs (27 described). The most frequently reported were fever (30.4%; n = 7),diarrhea (21.7%; n = 5), and erythema (21.7%; n = 5). Thirty-four different medications were involved (same active substance or fixed combinations), mainly antibacterials (n = 14; 41.2%) and vaccines (n = 13; 38.2%). After causality assessment, 11 (32.4%) were scored “possible,” 16 (47.1%) “plausible,” and 7 (20.6%) “likely.” All ADRs were labeled, except 1 (erythema with sun exposure, caused by amoxicillin). Evolution was favorable in all cases. None of the ADRs were considered serious, and all were reported to the regional pharmacovigilance center.
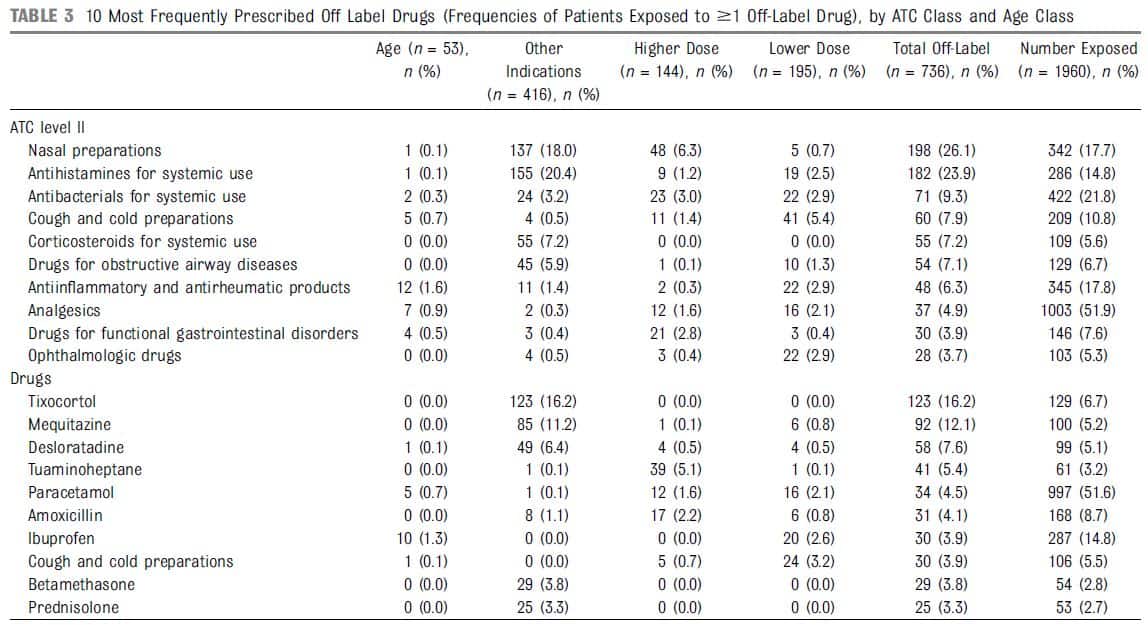
Total exposed: number of patients exposed to the corresponding substance. Number off label: number of patients exposed to ≥1 off-label prescription for the corresponding
substance.
Relationship Between Off-Label Prescribing and ADR Occurrence
Off-label prescribing was identified in 5 of 23 cases and involved prescribing for an unapproved indication in 3 cases (hydroxyzine and mequitazine for pruritus, betamethasone for pneumopathy) and an inadequate dosage of amoxicillin for the 2 remaining cases. Incidence of ADRs was 1.5% (11 in 736) for patients exposed to ≥1 off-label prescription and 1.0% in other patients. ADR occurrence was not related to off-label drug prescribing (crude OR 1.53; 95% CI, 0.67–3.49; P = .310). Multivariable analyses did not reveal significant associations.
Discussion
Despite the numerous initiatives implemented since 2006 to promote rational medicine use in children, off-label prescription in outpatient pediatric practice remains high (37.6%). However, in contrast to the findings of a previous study conducted >10 years ago, we did not find an increased ADR risk related to off-label prescribing.
Strengths and Limitations
One of the strengths of this study is its prospective design, enabling us to study the incidence of ADRs in our sample. We were able to record more detailed information on exposure to medications than in retrospective studies of medication use reported by parents or relatives. Moreover, unlike in studies performed on reimbursement databases, data on indications were available in our study, which permitted us to better characterize off label prescribing in several categories (eg, contraindication, different indication). This study is also one of the few studies in outpatient settings published since implementation of Pediatric Regulation No. 1901/2006. To assess representativeness of prescribing schemes, we compared data from reimbursement claims with prescribing patterns in our study. They exhibited strong similarities, except for paracetamol, which is not systematically reimbursed. Because participation was voluntary, the possibility of selection bias among GPs should be discussed. They were all involved in resident training and thus likely to be more aware of off-label prescribing, resulting in a possible underestimation of the real incidence. Concerning the completeness of ADR data, the possibility of underreporting should be considered very low, and serious ADRs would have been detected, because GPs are systematically informed in cases of hospitalization.
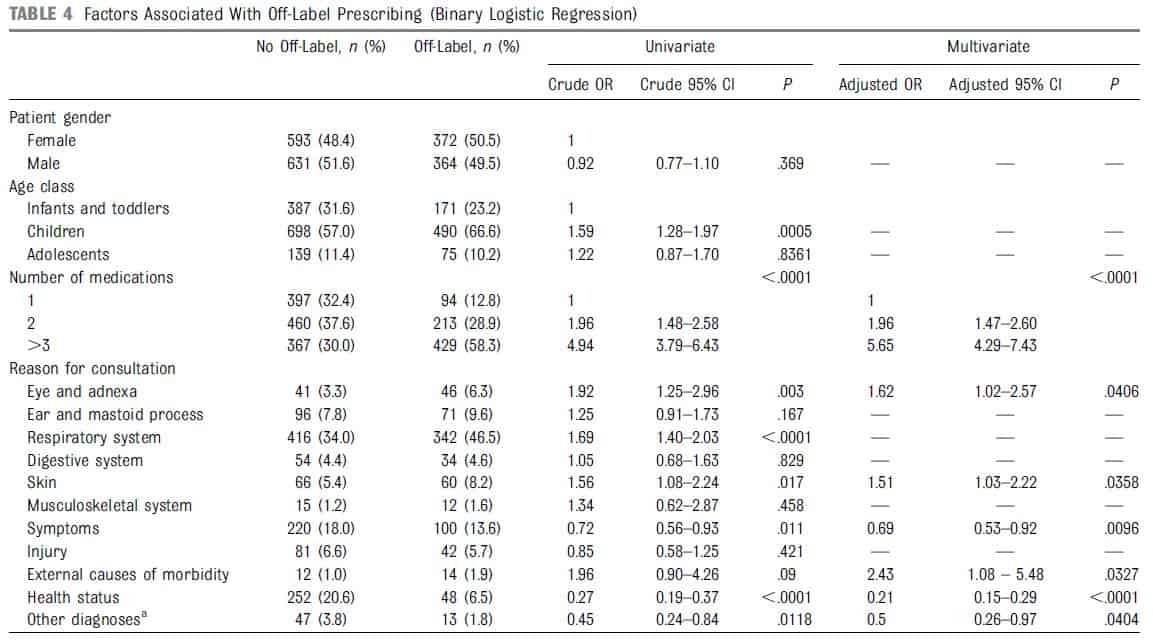
a Other diagnoses: blood, endocrine, nutritional and metabolic diseases, mental and behavioral disorders, nervous system, circulatory system, genitourinary system, and conditions
originating in the perinatal period.
Studies of Off-Label Prescribing in Outpatient Settings
The study performed in the same region in 2000 among pediatricians2 showed 42% off-label prescribing. When we compare diagnosis profiles, respiratory symptoms are still the most common but with a lower prevalence (38.7% vs 56.5% in 2000). Symptoms (eg, fever) were more common in our study (16.3% vs 11.5% in 2000), together with infections (13.6% vs 9.3% in 2000). We did not observe as many diagnoses of the nervous and sensory systems (0.5% vs 15.3% in 2000). The prevalence of factors influencing health status tended to be lower in our study (15.3% vs 28.7% in 2000). By contrast, patterns of prescribing did not differ extensively: Systemic corticosteroids were similar (5.6% vs 5.0% of children in 2000), and antibiotics (21.8% vs 26.8%), vaccines (13.1% vs 19.0%), and respiratory drugs (37.1% vs 48.6%) were less prescribed. The greatest difference was for paracetamol (51.6% vs 23.7%). Another French survey performed in the same period18 found 29% off-label or unlicensed prescribing, and a German survey performed in 2003 to 200619 found 40.2% off-label prescribing.
Studies performed between 2001 and 200720–22 on prescription databases revealed high off-label rates, from 13.5% to 62%, except an Italian study23 with a 3.3% rate. This heterogeneity in results could be explained by differences in off-label definitions. To ensure comparability, we use the same definitions as in our previous study.2
The most frequently retrieved classes of off-label prescriptions were nasal preparations (tixocortol, tuaminoheptane), H1 antihistamines for systemic use (mequitazine, desloratadine), and antibacterials (amoxicillin). In the study by Horen et al,2 corticoids and β2 mimetics for bronchiolitis, decongestants for rhinopharyngitis, and drugs from “blood forming organs” (prescribed to reinforce immunity in infectious ear, nose, and throat diseases) were the most frequently prescribed off label.
In the Swedish study,22 off-label prescribing involved mainly topically administered drugs, sex hormones, antidepressants, hypnotics, cardiovascular drugs, and nonsteroidal antiinflammatory drugs. The most common classes in the Italian study23 were antibiotics and drugs for the alimentary tract, metabolism, and the respiratory tract. However, the age groups were not strictly similar; the Swedish study22 included patients >16 years old. In our study, unlabeled indications, accounting for a large part of off-label prescribing, were higher in children (23.1%) than in infants (18.8%)
or adolescents (16.8%). The difference between children and infants is explained by the prescribing of desloratadine (76 [6.4%] of children vs 11 [2.1%] infants exposed, with 41 unlabeled indications in children vs 4 in infants). Tixocortol was the most frequently prescribed with unlabeled indications in children and infants. Adolescents, who were less exposed to tixocortol, exhibited
a lower rate of unlabeled indications related to this drug.
Studies Investigating ADR Occurrence in Outpatient Settings
Incidences observed in our study appear to be consistent with those reported in outpatient settings.8 In the systematic review performed by Smyth et al in 2012,24 studies in GP settings exhibited an ADR incidence between 0.75% and 1.41%.
Relationship Between Off-Label Prescribing and ADR Occurrence in Inpatient and Outpatient Settings
In our study, ADR occurrence was not significantly related to off-label prescribing. Other studies2,25–27 have shown conflicting results. In the Horen et al2 study, 60% of the ADRs involved an off-label prescription (compared with 48% in our study), with a significant association between off-label prescribing and ADR (relative risk 3.44; 95% CI, 1.26–9.38). These ADRs were related mainly to antibiotics and vaccines, which were prescribed less often in our study. Thus, we might suspect that the greater risk of ADRs observed by Horen resulted from a different pattern of off-label use, related to differences in prescribing patterns. Bellis et al26 concluded that off-label and unlicensed medicines were more likely to be involved in an ADR leading to hospitalization (relative risk 1.67; 95% CI, 1.38–2.02; P < .001). In a survey of pediatric wards, Neubert et al27 found no significant difference between ADRs in relation to off-label prescribing. A retrospective analysis of spontaneous reports in the Danish ADR database from 1998 to 2007 showed that about 20% of ADRs reported in Danish children over the study period were associated with off-label prescribing outside the licensed age group.25 As concluded in the review from Mason et al,28 it seems that there is no consistent evidence suggesting a greater risk related to off-label prescribing, perhaps because of the heterogeneity in prescribing settings or practices considered in the studies. Indeed, some studies found high rates of off-label prescribing of mental health or cancer medications.22,26 The risk reported by Bellis et al becomes not statistically significant when oncology patients were excluded from the analysis. In our study, GPs were not treating children with severe mental health problems, cardiac diseases, or cancer. It is therefore possible that ADR risk increases mainly with specific types of off-label use.
Perspectives
Unapproved indications were the main motive for off-label prescribing. In another study, underdosing was more represented.19 Beyond this observation, more information about the underlying causes of off-label prescribing is needed. In the present study, individual factors related to the number of medications or certain medical conditions were found to be associated with off-label prescribing. Other factors have already been highlighted.29 Determinants related to prescriber have also been described, such as lack of training or information. Moreover, it is generally believed that off-label prescribing is prompted by the unavailability of pediatric formulations. The typology of off-label prescribing for unapproved conditions tends to reveal the limitations of the prescribers’ choices, because it involved indications that were closely related to the labeled indications (salbutamol for bronchospasm in the context of infection, where asthma is the labeled indication).
Since the implementation of the first pediatric investigation plans in 2007,30,31 only a small proportion of the drugs frequently prescribed off-label have been covered by this regulation. Thus, the rate of off-label prescribing, even if not associated with ADR occurrence, should prompt more efforts to promote rational drug use and achieve change in prescribing patterns.32
Recently, the new policy statement from the American Academy of Pediatrics on off-label prescribing7 recommended that “the use of a drug, whether off or on label, should be based on sound scientific evidence, expert medical judgment, or published literature whenever possible.” This study was performed in a European context, and some drugs are not in general use in the United States (eg, helicidine, phloroglucinol, tiaprofenic acid). However, these drugs were neither the most commonly used nor the most involved in off-label prescribing. Most of the drugs prescribed off
label in the current study are used worldwide, and possible crossnational differences in the availability of some drugs are not likely to affect the relevance of our findings for pediatric practice. This study provides additional scientific evidence on off-label drug prescribing in a setting that was poorly investigated in the literature, and therefore it may be useful for both clinicians and policymakers.
Conclusions
Despite the numerous initiatives implemented since 2006 to promote rational medication use in children, the prevalence of off-label prescribing in outpatient pediatric practice remains high. In contrast with the findings of a previous study conducted >10 years ago, we did not find greater ADR risk related to off-label prescribing.
Acknowledgments
The authors are grateful for help from the department of general practice of Toulouse. The authors also thank all general practitioners and residents who contributed to this study.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: No external funding.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
1. Cuzzolin L, Atzei A, Fanos V
Off-label and unlicensed prescribing for newborns and children in different settings:
a review of the literature and
a consideration about drug safety.
Expert Opin Drug Saf. 2006;5(5):703–718
2. Horen B, Montastruc JL, Lapeyre-Mestre M.
Adverse drug reactions and off-label drug use in paediatric outpatients.
Br J Clin Pharmacol. 2002;54(6):665–670
3. Turner S, Nunn AJ, Fielding K, Choonara I.
Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr.
1999; 88(9):965–968
4. Star K, Edwards IR.
Pharmacovigilance for children’s sake.
Drug Saf. 2014;37(2): 91–98
5. Lalande J.
[Appel, a general pediatrics child welfare organization].
Arch Pediatr. 2003;10(suppl 1):245s–247s
6. Lehmann B. Regulation (EC)
No 1901/2006 on medicinal products for paediatric use & clinical research in vulnerable populations.
Child Adolesc Psychiatry Ment Health. 2008;2(1):37
7. Frattarelli DA, Galinkin JL, Green TP, et al;
American Academy of Pediatrics Committee on Drugs. Off-label use of drugs in children.
Pediatrics. 2014; 133(3):563–567
8. Clavenna A, Bonati M.
Adverse drug reactions in childhood: a review of prospective studies and safety alerts.
Arch Dis Child. 2009;94(9):724–728
9. Sorum PC.
Two tiers of physicians in France: general pediatrics declines, general practice rises.
JAMA. 1998; 280(12):1099–1101
10. Bocquet A, Chalumeau M, Bollotte D, Escano G, Langue J, Virey B.
Comparison of prescriptions by pediatricians and general practitioners: a populationbased study in Franche-Comte from the database of Regional Health Insurance Fund.
Arch Pediatr. 2005;12(12): 1688–1696
11. International Conference on Harmonisation (ICH).
ICH harmonised tripartite guideline E11: clinical investigation of medicinal products in the pediatric population.
2000. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E11/Step4/E11_Guideline.pdf
12. Fagot JP, Boutrelle A, Ricordeau P, Weill A, Allemand H.
HPV vaccination in France: uptake, costs and issues for the National Health Insurance.
Vaccine. 2011;29(19): 3610–3616
13. Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merlière Y.
French National Health Insurance information system and the permanent beneficiaries sample.
Rev Epidemiol Sante Publique. 2010; 58(4):286–290
14. Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM).
Répertoire des spécialités pharmaceutiques. Available at: http://agence-prd.ansm.sante.fr/php/ecodex/index.php.
Accessed March 12, 2014
15. Bégaud B, Evreux JC, Jouglard J, Lagier G.
[Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France].
Therapie. 1985; 40(2):111–118
16. Edwards IR, Aronson JK.
Adverse drug reactions: definitions, diagnosis, and management.
Lancet. 2000;356(9237): 1255–1259
17. Bonnes pratiques de pharmacovigilance.
Mise à jour prenant en compte l’arrêté du 10 juin 2011 relatif aux modalités de signalement des effets indésirables par les patients et les associations agréées de patients.
Available at: http://ansm.sante.fr/var/ansm_site/storage/original/application/13df5d1566a748c2f08299233451fe5c.pdf.
Accessed March 12, 2014
18. Chalumeau M, Tréluyer JM, Salanave B, et al.
Off label and unlicensed drug use among French office based paediatricians.
Arch Dis Child. 2000; 83(6):502–505
19. Knopf H, Wolf IK, Sarganas G, Zhuang W, Rascher W, Neubert A.
Off-label medicine use in children and adolescents: results of a populationbased study in Germany.
BMC Public Health. 2013;13(1):631
20. Bazzano AT, Mangione-Smith R, Schonlau M, Suttorp MJ, Brook RH.
Off-label prescribing to children in the United States outpatient setting.
Acad Pediatr. 2009;9(2):81–88
21. Lass J, Irs A, Pisarev H, Leinemann T, Lutsar I.
Off label use of prescription medicines in children in outpatient setting in Estonia is common.
Pharmacoepidemiol Drug Saf. 2011;20(5):474–481
22. Olsson J, Kimland E, Pettersson S, Odlind V.
Paediatric drug use with focus on offlabel prescriptions in Swedish outpatient care—a nationwide study.
Acta Paediatr. 2011;100(9):1272–1275
23. Carnovale C, Conti V, Perrone V, et al.
Paediatric drug use with focus on offlabel prescriptions in Lombardy and implications for therapeutic approaches.
Eur J Pediatr. 2013;172(12): 1679–1685
24. Smyth RM, Gargon E, Kirkham J, et al.
Adverse drug reactions in children—a systematic review.
PLoS ONE. 2012;7(3): e24061
25. Aagaard L, Hansen EH.
Prescribing of medicines in the Danish paediatric population outwith the licensed age group: characteristics of adverse drug reactions.
Br J Clin Pharmacol. 2011;71(5):751–757
26. Bellis JR, Kirkham JJ, Nunn AJ, Pirmohamed M.
Adverse drug reactions and off-label and unlicensed medicines in children: a prospective cohort study of unplanned admissions to a paediatric hospital.
Br J Clin Pharmacol. 2014;77(3):545–553
27. Neubert A, Dormann H, Weiss J, et al.
The impact of unlicensed and off-label drug use on adverse drug reactions in paediatric patients.
Drug Saf. 2004; 27(13):1059–1067
28. Mason J, Pirmohamed M, Nunn T.
Off-label and unlicensed medicine use and adverse drug reactions in children: a narrative review of the literature.
Eur J Clin Pharmacol. 2012;68(1):21–28
29. Schirm E, Tobi H, de Jong-van den Berg LT.
Risk factors for unlicensed and off-label drug use in children outside the hospital.
Pediatrics. 2003;111(2):291–295
30. Haslund-Krog S, Mathiasen R, Christensen HR, Holst H.
The impact of legislation on drug substances used off-label in paediatric wards—a nationwide study.
Eur J Clin Pharmacol. 2014;70(4):445–452
31. Olski TM, Lampus SF, Gherarducci G, Saint Raymond A.
Three years of paediatric regulation in the European Union.
Eur J Clin Pharmacol. 2011;67(3):245–252
32. Milne CP, Davis J.
The pediatric studies initiative: after 15 years have we reached the limits of the law?
Clin Ther. 2014;36(2):156–162
www.pediatrics.org/cgi/doi/10.1542/peds.2014-0764
DOI: 10.1542/peds.2014-0764
Accepted for publication Oct 9, 2014
Address correspondence to Maryse Lapeyre-Mestre, MD, PhD, University of Toulouse UMR INSERM 1027, CHU, 37 Allées Jules Guesde, 31000 Toulouse, France. E-mail: maryse.lapeyre-mestre@univ-tlse3.fr
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2015 by the American Academy of Pediatrics
